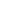
| Registration at Glance | |||
| SR. NO | Name of Country | No. of Products Registered | No. of Products under Registration |
| 1 | MOZAMBIQUE | 56 | 23 |
| 2 | CAMBODIA | 14 | — |
| 3 | UGANDA | 11 | 17 |
| 4 | TAJIKISTAN | 10 | 4 |
| 5 | CONGO | 6 | — |
| 6 | TANZANIA | 2 | 6 |
| 7 | ZAMBIA | 2 | 10 |
| 8 | MYANMAR | 2 | 13 |
| 9 | VIETNAM | 1 | — |
| 10 | CAMEROON | — | 6 |
| 11 | MALI | — | 3 |
| 12 | NIGER | — | 6 |
| 13 | TCHAD | — | 5 |
| 14 | COSTA RICA | — | 2 |
| 15 | PHILLIPINES | 9 | — |
 022-25624922
022-25624922